Abstract
Background: Chemotherapy (CT)-induced neutropenia is a common complication from chemotherapy, limiting optimal dosing and treatment. Tbo-filgrastim (GRANIX®) is a non-glycosylated recombinant methionyl human granulocyte colony-stimulating growth factor manufactured by recombinant DNA technology. It is indicated to reduce the duration of severe neutropenia (SN) in patients with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia (FN). It is approved under a biologic license application in the United States. This phase 2, multicenter, open-label study investigated the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and immunogenicity of tbo-filgrastim in pediatric patients receiving at least 1 cycle of myelosuppressive CT.
Methods: Patients ages 1 mo to <16 y with solid tumors without bone marrow involvement were administered tbo-filgrastim 5 µg/kg/body weight once daily subcutaneously. Tbo-filgrastim administration was started at approximately 24 hours (±3 hours) after the end of the last CT treatment. Daily dosing with tbo-filgrastim continued until the expected neutrophil nadir was passed and the neutrophil count had recovered to 2.0 × 109/L but not longer than 14 consecutive days. Patients were stratified into three age groups: infants (1 mo to <2 y), children (2 to <12 y), and adolescents (12 to <16 y). The CT regimens included at least one of the following: etoposide, doxorubicin, ifosfamide, or cyclophosphamide. The primary endpoint was the safety of tbo-filgrastim; secondary objectives were to assess the PK, PD (incidence and duration of SN, defined as absolute neutrophil count <0.5x109/L), and efficacy (incidence of FN). Immunogenicity was also assessed.
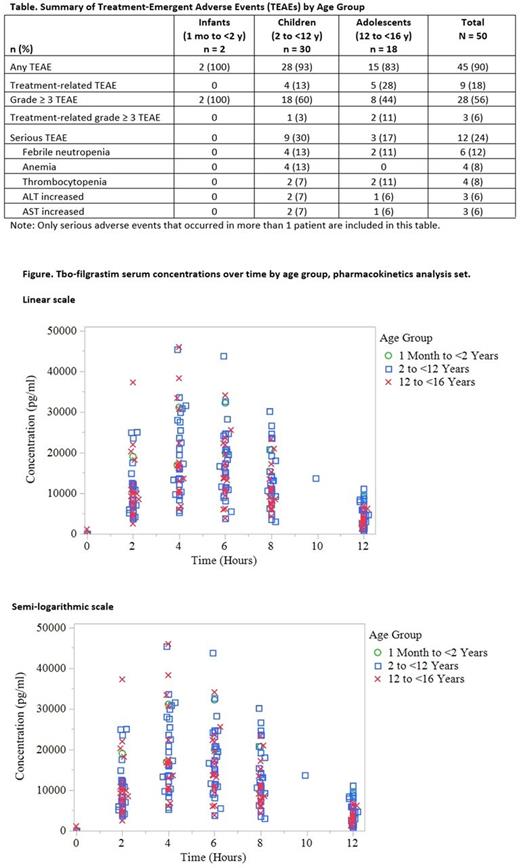
Results: Fifty patients (2 infants, 30 children, and 18 adolescents) were administered a single daily dose of tbo-filgrastim. The most common cancers were rhabdomyosarcoma (14%), neuroblastoma (14%), Ewing tumors (12%), and osteosarcoma (12%). The mean (SD) number of doses administered were 9.2 (2.83) in children and 7.3 (1.88) in adolescents. Twelve and 14 doses, respectively, were administered to each infant. Serious treatment-emergent adverse events (TEAEs) were reported in 24% of patients (Table). The most common serious TEAEs were FN (12%, 4 children, 2 adolescents), anemia (8%, 4 children) and thrombocytopenia (8%, 2 children, 2 adolescents), increased alanine aminotransferase (ALT) and increased aspartate aminotransferase (AST) (6% each). The majority of grade 3 and 4 events, per Common Terminology Criteria for Adverse Events (NCI CTCAE), were in the hematologic system organ class. Nine patients (18%) experienced treatment-related TEAEs. The most common treatment-related TEAEs were musculoskeletal and connective tissue disorders (4/50, 8%) and were of NCI CTCAE grade 1 severity. Two patients (2/50, 4%) had increases in liver function enzymes considered to be related to tbo-filgrastim and CT by the investigator. No clinical symptoms were observed with these events, which resolved by the end of the study. No deaths or study withdrawal occurred during the study. PK parameters of exposure were comparable between age groups as shown by the overlap of tbo-filgrastim serum concentrations (Figure). The incidence of SN was 52% (26/50; 95% CI 0.374-0.663). The mean (SD) duration of SN was 1.8 (2.21) d. The incidence of FN was 26% (13/50; 95% CI 0.146-0.403). Immunogenicity assessments found that none of the patients had an antidrug antibody response after tbo-filgrastim treatment.
Conclusion: A daily dose of tbo-filgrastim 5 μg/kg/body weight administered to pediatric patients with solid tumors without bone marrow involvement demonstrated a safety profile consistent with the safety profile in adult patients. No immunogenic response was observed in this population. The increases in liver function enzymes were considered related to both tbo-filgrastim and CT by the investigator. The sponsor assessed these events to be related to CT and concomitant medications and not related to tbo-filgrastim. The incidence of FN was on the lower end of the range in the literature. PD variables of the incidence and duration of SN provide supportive results of the efficacy of tbo-filgrastim in pediatric cancer patients.
Bias: Teva ratiopharm: Employment, Equity Ownership. Lammerich: Teva ratiopharm: Employment, Equity Ownership. Roth-Ben Arie: Teva Pharmaceuticals Industries: Employment. Zou: Teva Pharmaceuticals: Employment, Equity Ownership. Buchner: Teva ratiopharm: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.